By John Lamb, Fabian Fernandez and Daniel Kaiser, Extension Specialists in Nutrient Management
Environmental and economic issues have increased the need to better understand the role and fate of nitrogen (N) in crop production systems. Nitrogen is the nutrient most often deficient for crop production in Minnesota, and its use can result in substantial economic return for farmers. However, when N inputs to the soil system exceed crop needs, there is a possibility that excessive amounts of nitrate (NO3-N) may enter either ground or surface water.
Managing N inputs to achieve a balance between profitable crop production and environmentally tolerable levels of nitrate in water supplies should be every grower’s goal. The behavior of N in the soil system is complex, yet an understanding of these basic processes is essential for a more efficient N management program.
Nitrogen Cycle
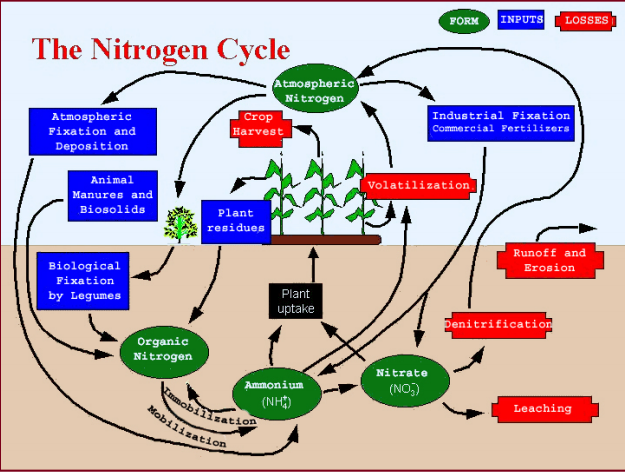
Nitrogen exists in the soil system in many forms and changes very easily form one form to another. The route that N follows in and out of the soil system is collectively called the “N cycle” (see photo).
The nitrogen cycle is biologically influenced. Biological processes, in turn, are influenced by prevailing climatic conditions along with the physical and chemical properties of a particular soil. Both climate and soils vary greatly across Minnesota and affect the N transformations for the different areas.
Inputs of N for Plant Growth
- Atmosphere
- Biological fixation
- Atmospheric fixation
- Precipitation (deposition)
- Industrial fixation — commercial fertilizers
- Soil organic matter
- Crop residues
- Animal manures
Atmospheric N is the major reservoir for N in the N cycle (air is 79% N2 gas). Although unavailable to most plants, large amounts of N2 can be used by leguminous plants via biological N fixation. In this biological process, nodule-forming Rhizobium bacteria inhabit the roots of leguminous plants, and through a symbiotic relationship, convert atmospheric N2 to a form the plant can use.
The amount of N2 fixed by legumes into usable N can be substantial, with a potential for several hundred pounds N per acre per year to be fixed in an alfalfa crop. Any portion of a legume crop that is left after harvest, including roots and nodules, can supply N to the soil system when the plant material is decomposed.
Several non-symbiotic organisms exist that fix N, but N additions from these organisms are quite low (1-5 pounds per acre per year). In addition, small amounts of N are added to soil from precipitation. The amount of N supplied from precipitation averages 5-10 pounds per acre per year in Minnesota.
Commercial N fertilizers are also derived from the atmospheric N pool. The major step is to combine N2 with hydrogen (H2) to form ammonia (NH3). Anhydrous ammonia is then used as a starting point in the manufacture of other N fertilizers.
Anhydrous ammonia or other N products derived from ammonia can then supplement other N sources for crop nutrition. Nitrogen can also become available for plant use from organic N sources. But first these organic sources must be converted to inorganic forms before they are available to plants. Nitrogen is available to plants as either ammonium (NH4+-N) or nitrate.
Animal manures and other organic wastes can be important sources of N for plant growth. The amount of N supplied by manure will vary with the type of livestock, handling, rate applied and method of application. Since the N form and content of manures vary widely, an analysis of manure is recommended to improve N management.
Crop residues from non-leguminous plants also contain N, but in relatively small amounts compared with legumes. Nitrogen exists in crop residues in complex organic forms and the residue must decay (a process that can take several years) before N is made available for plant use.
Soil organic matter is also a major source of N used by crops. Organic matter is composed primarily of rather stable material called humus that has collected over a long period of time. Easily decomposed portions of organic material disappear relatively quickly, leaving behind residues more resistant to decay. Soils contain approximately 2,000 pounds N in organic forms for each percent of organic matter. Decomposition of this portion of organic matter proceeds at a rather slow rate and releases about 20 pounds of N per acre per year for each percent of organic matter. A credit for the amount of N released by organic matter is built into current University of Minnesota N guidelines.
Nitrogen Transformations
Nitrogen, present or added to the soil, is subject to several changes (transformations) that dictate the availability of N to plants and influence the potential movement of nitrates to water supplies.
Organic N that is present in soil organic matter, crop residues and manure is converted to inorganic N through the process of mineralization. In this process, bacteria digest organic material and release ammonium. Formation of ammonium increases as microbial activity increases. Bacterial growth is directly related to soil temperature and water content.
The ammonium supplied from fertilizer is the same as the ammonium supplied from organic matter. Ammonium-N has properties that are of practical importance for N management. Plants can absorb ammonium. Ammonium also has a positive charge and therefore is attracted or held by negatively charged soil and soil organic matter. This means that ammonium does not move downward in soils. Nitrogen in the ammonium form that is not taken up by plants is subject to other changes in the soil system.
Nitrification is the conversion of ammonium to nitrate. Nitrification is a biological process and proceeds rapidly in warm, moist, well aerated soils. Nitrification slows at soil temperatures below 50 F. Nitrate-N is a negatively charged ion and is not attracted to soil particles or soil organic matter like ammonium. Nitrate-N is water soluble and can move below the crop rooting zone under certain conditions.
Denitrification is a process by which bacteria convert nitrate to N gases that are lost to the atmosphere. Denitrifying bacteria use nitrate instead of oxygen in the metabolic processes. Denitrification takes place in waterlogged soil and with ample organic matter to provide energy for bacteria. For these reasons, denitrification is generally limited to topsoil.
Denitrification can proceed rapidly when soils are warm and become saturated for 2 or 3 days. A temporary reduction in the mount of plant available N can occur from immobilization (tie up) of soil N. Bacteria that decompose high carbon-low N residues, such as corn stalks or small grain straw, need more N to digest the material than is present in the residue.
Immobilization occurs when nitrate and/or ammonium present in the soil is used by the growing microbes to build proteins. The actively growing bacteria that immobilize some soil N also break down soil organic matter to release available N during the growing season. There is often a net gain of N during the growing season because the additional N in the residue will be the net gain after immobilization-mineralization processes.
Nitrogen Loss from the Soil System
- Leaching
- Denitrification
- Volatilization
- Crop removal
- Soil erosion and runoff
In contrast to the biological transformations previously described, loss of nitrate by leaching is a physical event. Leaching is the loss of soluble nitrate as it moves with soil water, generally excess water, below the root zone. Nitrate-N that moves below the root zone has the potential to enter either groundwater or surface water through tile drainage systems.
Coarse-textured soils have a lower water-holding capacity and, therefore, a greater potential to lose nitrate from leaching when compared with fine-textured soils. Some sandy soils, for instance, may retain only ½ inch of water per foot of soil while some silt loam or clay loam soils may retain up to 2 inches of water per foot.
Nitrate-N can be leached from any soil if rainfall or irrigation moves water through the root zone. Denitrification can be a major loss mechanism of nitrate when soils are saturated with water for 2 or 3 days. Nitrogen in the ammonium form is not subject to this loss.
Management alternatives are available if denitrification losses are a potential problem. Significant losses from some surface-applied N sources can occur through the process of volatilization. In this process, N is lost as ammonia gas. Nitrogen can be lost in this way from manure and fertilizer products containing urea. Ammonia is an intermediate form of N during the process in which urea is transformed to ammonium.
Incorporation of these N sources will virtually eliminate volatilization losses. Loss of N from volatilization is greater when soil pH is higher than 7.3, the air temperature is high, the soil surface is moist and there is a lot of residue on the soil.
Substantial amounts of N are lost from the soil system through crop removal. A 250-bushel per acre corn crop, for example, removes approximately 175 pounds of N with the grain. Crop removal accounts for a majority of the N that leaves the soil system.
Nitrogen can be lost from agricultural lands through soil erosion and runoff. Losses through these events do not normally account for a large portion of the soil N budget, but should be considered for surface water quality issues. Incorporation or injection of manure and fertilizer can help to protect against N low through erosion or runoff. Where soils are highly erodible, conservation tillage can reduce soil erosion and runoff, resulting in less surface low of N.
Avoid Misconceptions
In considering the many transformations and reactions of N in soils, there are some major points to keep in mind. Although N can be added to soil in either organic or inorganic forms, plants take up only inorganic N (that is nitrate and ammonium). One form is not more important than the other and all sources of N can be converted to nitrate. Commercial N fertilizers, legumes, manures and crop residues are all initial sources of nitrate and ammonium, and once in the plant or in the water supply it is impossible to identify the initial source.
Nitrate is always present in the soil solution and will move with the soil water. Inhibiting the conversion of ammonium to nitrate can result in less N loss and more plant uptake; however, it is not possible to totally prevent the movement of some nitrate to water supplies, but sound management practices can keep losses within acceptable limits.
Summary
This article discusses several factors that are critical for understanding N behavior in a soil system. Numerous sources of N exist and must be considered when evaluating the N budget for any field or region. Nitrogen’s mobility factor in the soil must be considered when developing N programs and evaluation environmental effects. Nitrogen loss from the soil system is greatly affected by soil type and climate. Sandy soils may lose N through leaching, while on heavy, poorly drained soils it may be lost through denitrification. Because Minnesota has such diverse soils and climate, interpretation of the N cycle should be site specific.






Post a comment
Report Abusive Comment