The carefully calculated and measured rate of glyphosate that goes in the spray tank is the same deadly dose that makes it to weed leaves, right?
Not necessarily, says Murray State University chemistry professor Scott Brown, who’s spent 4 years studying issues that surround the effectiveness of glyphosate.
Raised on a Kentucky farm, Brown began to investigate herbicide molecules and their activity while working on his degrees and managing his own farming operation.
He’s found that no-tillers with hard water may be losing some, or even most of their glyphosate activity before they even head for the fields — and those that think they’re already addressing the issue might still be missing the mark.
Essentially, no-tillers with hard water may be unknowingly spraying a lesser rate and getting a reduced response because of how glyphosate interacts with the minerals in the water, says Brown, who earned an undergraduate degree in chemistry and agriculture from Western Kentucky University and a doctorate from the University of Alabama.
Besides being a wasted herbicide investment, this interaction can be a recipe for weed disaster.

“The trend I’m seeing is that regions with hard water are having higher incidence of glyphosate-resistant weeds,” Brown told no-tillers at the National No-Tillage Conference earlier this year. “I’m not promising you that I can kill resistant pigweed or resistant marestail by addressing hard-water issues. But we’re losing activity right out of the gate.”
Brown became aware of the lack of knowledge about hard water, and its impact on the effectiveness of herbicides, when consulting with a farmer in the boot heel of Missouri. Despite what the farmer had been advised by his chemical supplier, the obvious fix to his herbicide efficacy problem was evident to Brown.
“The farmer had no idea what his water makeup was. The water had a hardness factor of greater than 250 milligrams per liter (mg/L) of calcium and he was given no recommendation to manage the water prior to introduction of chemical,” Brown says.
The calcium was taking the punch right out of his herbicides, creating an easy path for resistant weeds and the farmer simply didn’t know it was an issue, Brown says, adding that the absence of knowledge transfer to farmers on this topic is a huge issue.
“It’s the biggest reason I began pursuing, researching and educating about the impact of water quality on spraying,” he says.
Chemistry 101
Grasping why hard water and glyphosate don’t play well together requires a basic chemistry lesson, including how glyphosate works.
Glyphosate is a chelation agent, but what does that really mean?
“Glyphosate is a metal attractor,” Brown explains. “If an element forms a 2+ cation, glyphosate will attract to it.”
Magnesium, zinc and iron, to name a few, all form 2+ cations, and once glyphosate hooks up with one of those metals, it never lets go. This is a positive development when glyphosate has entered a target plant. Once in the plant, glyphosate binds to these metal centers and blocks the plant from performing essential functions.
This tie-up of metals blocks schimate enzymes, and that’s why glyphosate is known as schimate inhibitor, Brown says.
Schimate enzymes are responsible for making amino acids, which make proteins that do everything, including build plant structure, fight off disease, and carry out normal biological processes for the plant.
These enzymes and amino acids are responsible for a plant’s natural defense mechanism, which is where glyphosate gains its advantage, Brown says.
“It’s like giving a plant AIDS,” Brown says. “Glyphosate doesn’t actually kill the plant. It works by tying up the metals a plant needs to make the amino acids and proteins to defend itself. The plant becomes vulnerable to other diseases that come and wipe it out.”
Plants form resistance by figuring out another way to produce amino acids and fight off diseases. It’s a fairly well-circulated warning that spraying too low of a rate of glyphosate can speed along resistance, Brown says.
In the case of hard water, the problem is that farmers may believe they’re spraying with a full rate but unknowingly creating a problem.
Hard water is an issue because hardness, he says, is a measure of the minerals in soluble form in water. Those minerals are predominantly calcium (Ca2+), magnesium (Mg2+) and iron (Fe2+) — all the 2+ cations that glyphosate wants to latch onto. But, of the three, calcium is the biggest problem, Brown says.
Glyphosate is a phosphonate — a cousin to a phosphate — and phosphates have a higher affinity for calcium than sulfates or sulfonates. When glyphosate is added to hard water it binds quickly to the calcium due to that affinity. And once grouped, they aren’t too keen on parting.
“The glyphosate is tied up by the calcium in the hard water and becomes inactive,” Brown says. “Once they’re bound together, the structure is super stable. It would take heat, sulfuric acid, phosphoric acid and days of time to get it to open back up.”
Hard Costs
The number of glyphosate molecules tied up by calcium depends on the number of calcium ions (the hardness of the water), the pH, and the amount of water used.
Brown is working with a technology called nuclear magnetic resonance (NMR), the same concept used in an MRI, to measure the rate and speed at which calcium consumes glyphosate.
“We’ve found that glyphosate molecules bind to an equal amount of calcium molecules, and the consumption happens in milliseconds,” Brown says.
But he notes other researchers have found that pH can have an impact on how much glyphosate is grabbed up by a single calcium molecule. At a pH of 8, for example, researchers have found that an astonishing four glyphosate molecules can bind to a single calcium molecule.
“Either way, as soon as you dump glyphosate into hard water, it’s dead,” Brown says.
Using a 1:1 tie-up ratio, Brown can calculate glyphosate losses at different water volumes and water-hardness levels. At a spray rate of 10 gallons per acre, and one glyphosate molecule binding to one calcium molecule, there is about a 3% loss of glyphosate molecules at a mid-moderate hardness level of 100 parts per million (ppm) calcium, Brown calculates.
At 1,000 ppm — very hard water — the glyphosate loss is 30%.
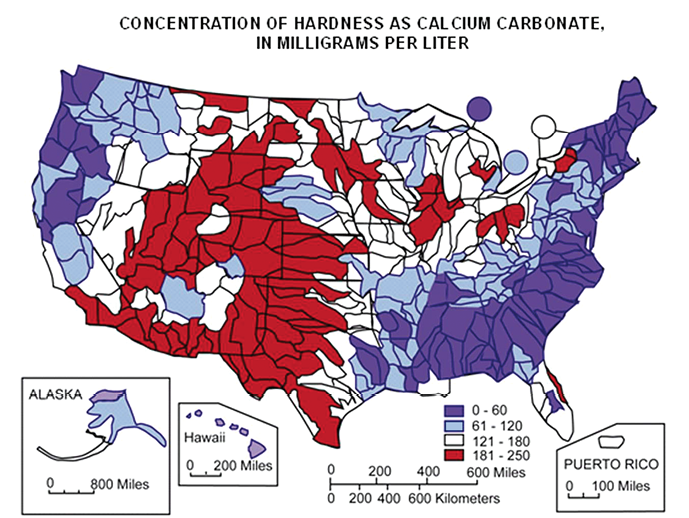
Many farmers think their water isn’t very hard and they’re fine to spray without worrying about glyphosate losses, Brown says.
“Acceptable water hardness when using glyphosate should be below 20 ppm, and a pH of 5, if farmers don’t want to use something like ammonium sulfate (AMS) to soften the water,” Brown says. “I doubt many farmers have that kind of water.
“What’s a good percent to lose of your glyphosate rate? Is 10% okay? How about 20%? The answer is 0%. We should all be addressing hard water issues.”
Hardness Or pH?
“I’ve heard world-renowned weed scientists tell farmers they need to check the hardness pH of their water,” Brown says. “I’m a chemist and I can’t for the life of me figure out what hardness pH is. That’s like saying apple orange or football bat. It just doesn’t make sense.”
Brown says water pH and hardness are actually completely independent terms and are independent measurements. While pH readings may indicate water is ideal for spraying, the hardness factor could be off the charts, rendering glyphosate useless. Both should be measured and dealt with accordingly.
No-tillers can get an easy-to-use handheld water hardness meter (total dissolved solids detector) for about $20 by ordering one off the Web. For pH, Brown recommends using pH strips as opposed to litmus paper because litmus paper can deteriorate and lose its effectiveness over time. Brown says it’s a good idea to have a lab test the water every year, if not more often, to get a total perspective of the hardness and pH.
Sizing Up Workarounds
If no-tillers don’t feel their glyphosate applications are working as well as they used to, there are several recommendations commonly given by industry representatives that might help farmers push efficacy a little harder, Brown says.
But if hard water is the problem, he adds, most of these strategies won’t work, and at least one of them hurts more than helps.
1. Add More Water. Some experts recommend bumping spray rates to 15, 20 or even 25 gallons per acre.
That’s a bad idea, Brown says.
“If hard water is the problem, the only thing adding more water does is increase the amount of calcium there is for glyphosate to bond with and be rendered useless,” he says. “At 1,000 ppm water hardness and a 25-gallon spray volume, glyphosate effectiveness would be reduced to 24% — not to mention time lost to constantly refilling the spray tank. The weeds will just roll a little and then rebound even more vigorously.”
Even at a more moderate hard-water level of 100 ppm, glyphosate effectiveness would be reduced by 5% when spray volume is bumped to 15 gallons per acre.
2. Change Nozzles. The theory here is getting a finer spray droplet to the leaf will make the chemical absorb better. But this won’t accomplish anything if glyphosate has already been taken out of commission.
3. Buy A New Sprayer. “If your glyphosate is dead in the water because it’s tied up with calcium, I can take my pieced-together pull-type sprayer that we built on our farm and do just as good of job as a brand new $350,000 self-propelled sprayer,” Brown says.
Spray operators have to address the hard-water problem before these strategies can be implemented to any benefit.
4. Reverse Osmosis Fix. While reverse osmosis (RO) systems do reduce or eliminate the hardness problem, the pH of the water hasn’t been managed. So it’s necessary to add a chemical agent to reduce the pH, Brown says. The typical acidifying agent will also tie up hard water.
“Adding an RO system simply adds another step that will still require adjusting pH. It’s reasonable to assess that the RO system is a waste of time and money at that point,” he says.
5. Add AMS. This tip actually can help, but it has to be done correctly, Brown says, adding that the trick is for no-tillers to treat their water supply, not the water in the sprayer.
Where most no-tillers fall into a trap is using an induction tank. If the supply water hasn’t been treated and glyphosate is exposed to hard water in the tank, glyphosate is dead before it even hits the sprayer, Brown says.
“You need to pre-treat your water in your supply tank with AMS or another softening agent,” Brown says.
On his own farm, Brown adds a softening agent (So-il Boost Plus in his case) to his supply tank before he fills it, and lets it sit for an hour.
“I’ve had guys say they can’t wait that long, but if they’re filling a 6,000-gallon semi-truck tank it’s going to take a couple hours, and if they started with the softening agent in the tank it’s doing its job during that time,” he says.
Agitation is also important, especially when using dry formulations of AMS, to make sure the softening agent has many opportunities to collide with the calcium in the water.
“A softening agent, such as AMS, needs to be used at least an hour before adding glyphosate,” Brown says. “You should never expose glyphosate to water that hasn’t been treated. Glyphosate should be the last thing added to the spray tank.”
And don’t drop the ball when hiring a custom applicator. When Brown has custom spraying done on his farm, he’s sure to supply his own pre-treated water to make sure there aren’t any mistakes.
6. Use AMS Alternative. Brown notes that in his opinion there is value to be had in using the AMS alternative, such as So-il Boost Plus.
“It’s exactly what a chemist would put in to treat the water,” he says. “I’m not trying to sell it or anything, but if I were designing a product from the chemistry standpoint it would be So-il Boost plus, it just works.”
AMS is a soluble salt, so it’s not the best approach for acidifying water, he says, and has been known to cause yield drag in soybeans.
So-il Boost Plus, manufactured by SO-IL Service, will soften and correct the pH and give no-tillers the added benefits of increasing the solubility of their herbicides in water.
The company’s literature says 1 pint per acre of So-il Boost Plus will bring water pH to 5 and hold it, while buffering hard water and improving translocation of herbicides.
Another area to consider is the addition of a surfactant. Brown recommends plant-oil based surfactants like Land Oil or methylated seed oil.
“It’s like liquid needles,” he says. “It’ll protect the glyphosate and inject the herbicide right into the plant.”
A Southern Illinois University study showed that weed control with So-il Boost Plus controlled waterhemp 7% better than AMS alone, and morning glory 5% better.

Ready The Sprayer
If a no-tiller hasn’t been using softened water, the sprayer itself can be a source of contamination.
Brown recommends doing a good sprayer clean out as part of the switch to using a softened water supply.
“Use 10 or 15 gallons of windshield washer fluid to wash out the sprayer and the booms. Put 100 gallons of softened water in and flush it out really well. You’ll still have some residual hardness there, but we’re talking 0.10%,” Brown says. “When you refill with pretreated water it should handle any remaining hard water.
“With a clean sprayer and treated water, you should see at least 50% better performance. I don’t mean you’ll kill any more weeds, but you’re going to kill weeds faster. It’s going to do a vastly better job.”








Post a comment
Report Abusive Comment